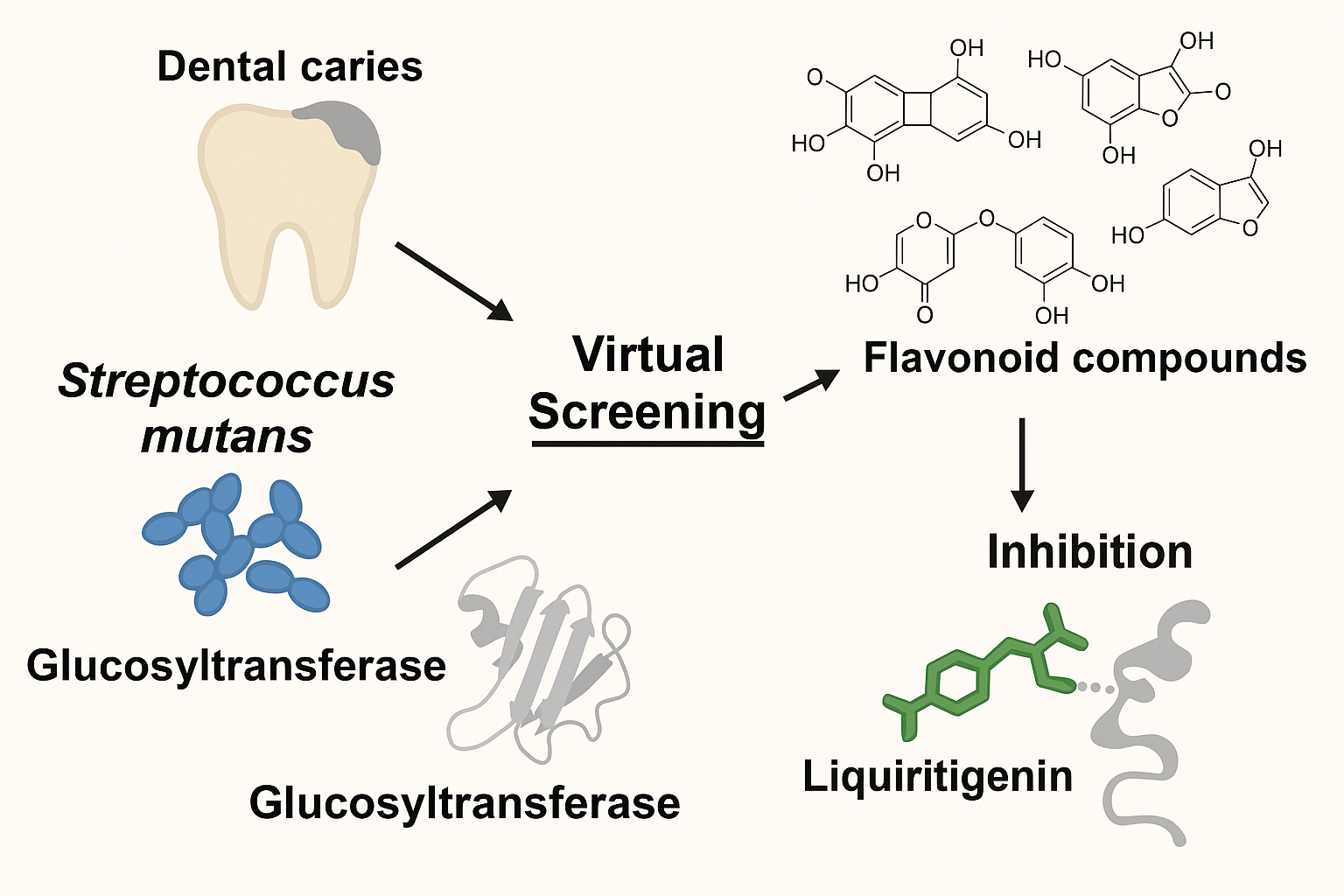
Virtual Screening of Flavonoid Compounds as Potential Antibiofilm Agents Targeting Glucosyltransferase
Abstract
Dental caries are caused by plaque formation resulting from biofilm accumulation on tooth surfaces. The bacterium Streptococcus mutans plays a crucial role in biofilm formation, partly through the production of glucosyltransferase, which catalyzes biofilm synthesis. Flavonoids are secondary metabolites commonly found in plants, known for diverse biological properties, including antibiofilm effects. This study aimed to screen the potential of flavonoid compounds as antibiofilm agents through inhibition of glucosyltransferase using an in-silico approach. A total of 87 flavonoid compounds obtained from the ZINC database were evaluated via molecular docking methods. Screening results based on binding free energy (ΔG) values, analyzed using the PyRX-Virtual Screening Tool, indicated that 36 compounds had potential to inhibit glucosyltransferase. Further molecular docking using AutoDock Vina identified nine compounds with ΔG values more favorable than the natural ligand of glucosyltransferase (maltose). Molecular interaction analysis using LigPlot+ and PyMOL revealed that taxifolin, gallocatechin, and sakuranetin interacted with three catalytic residues of the enzyme, whereas the remaining six compounds interacted with two catalytic residues. Liquiritigenin exhibited the lowest ΔG (-7.0 kcal/mol) and an inhibition constant (Ki) of 7.39 µM, indicating high affinity for glucosyltransferase. This compound formed two hydrogen bonds and four hydrophobic interactions, engaging two catalytic residues of the enzyme, Asn481 and Trp517. These findings highlight the potential of flavonoids as antibiofilm agents via glucosyltransferase inhibition. Further experimental validation through in vitro studies is necessary to confirm these in-silico findings.
Full text article
References
Agistia, D. D., Tegar, M., & Nugroho, A. E. (2013). Interaction between active compounds from Aegle marmelos Correa as anti-inflammatory agent with COX-1 and COX-2 receptor. Traditional Medicine Journal, 18(2), 80–87.
Amanda, Kunarti, S., & Subiwahyudi, A. (2017). Daya hambat aktivitas enzim glukosiltransferase (Gtf) Streptococcus mutans oleh ekstrak temulawak (Curcuma xanthorrhiza Roxb.). Conservative Dentistry Journal, 7(1), 32–36.
Amelia, F. (2014). Studi interaksi ligan peptinoid dan peptida dengan enzim protease NS3/NS2B virus dengue. Jurnal Saintek, 1(1), 24–29.
Arwansyah, & Hasrianti. (2014). Simulasi molecular docking senyawa kurkumin dan analognya sebagai selective androgen receptor modulators (SARMs) pada kanker prostat. Jurnal Dinamika, 5(2), 60–75.
Atta, L., Mushtaq, M., Siddiqui, A. R., Khalid, A., & Ul-Haq, Z. (2024). Targeting glucosyltransferases to combat dental caries: Current perspectives and future prospects. International Journal of Biological Macromolecules, 278(Pt 2), 134645. https://doi.org/10.1016/j.ijbiomac.2024.134645
Chen, D., Oezguen, N., Urvil, P., Ferguson, C., Dann, S. M., & Savidge, T. C. (2016). Regulation of protein–ligand binding affinity by hydrogen bond pairing. Science Advances, 2(3), e1501240. https://doi.org/10.1126/sciadv.1501240
Dallakyan, S., & Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. In H. B. & J. W. (Eds.), Methods in molecular biology (Vol. 1263, pp. 243–250). Humana Press. https://doi.org/10.1007/978-1-4939-2269-7_19
DeLano, W. L. (2009). PyMOL: An open-source molecular graphics tool. DeLano Scientific.
Du, X., Li, Y., Xia, Y. L., Ai, S. M., Liang, J., Sang, P., Ji, X. L., & Liu, S. Q. (2016). Insights into protein–ligand interactions: Mechanisms, models, and methods. International Journal of Molecular Sciences, 17(2), 144. https://doi.org/10.3390/ijms17020144
Egi, M., Soegiharto, G. S., & Evacuasiany, E. (2018). Efek berkumur sari buah tomat (Solanum lycopersicum L.) terhadap indeks plak gigi. SONDE, 3(2), 70–85. https://doi.org/10.28932/sod.v3i2.1784
Fakhruri, M., Rahmayanti, Y., & Isfanda. (2021). Potensi fitokimia Citrus aurantium (hesperetin, naringenin) dalam menghambat xantin oksidase pada hiperurisemia secara in-silico. Jurnal Health Sains, 2(1), 79–89.
Fitriana, N., Lestari, S. R., & Lukiati, B. (2018). Senyawa alami bawang putih tunggal sebagai inhibitor LpxC bakteri Pseudomonas aeruginosa melalui virtual screening. Jurnal Kedokteran dan Kesehatan, 18(1), 25–33.
Gao, Z., Chen, X., Wang, C., Song, J., Xu, J., Liu, X., Qian, Y., & Suo, H. (2024). New strategies and mechanisms for targeting Streptococcus mutans biofilm formation to prevent dental caries: A review. Microbiological Research, 278, 127526. https://doi.org/10.1016/j.micres.2023.127526
Hassan, A. M., Gattan, H. S., Faizo, A. A., Alruhaili, M. H., Alharbi, A. S., Bajrai, L. H., Al-Zahrani, I. A., Dwivedi, V. D., & Azhar, E. I. (2024). Evaluating the binding potential and stability of drug-like compounds with the monkeypox virus VP39 protein using molecular dynamics simulations and free energy analysis. Pharmaceuticals, 17(12), 1617. https://doi.org/10.3390/ph17121617
Irwin, J. J., Sterling, T., Mysinger, M. M., Bolstad, E. S., & Coleman, R. G. (2012). ZINC: A free tool to discover chemistry for biology. Journal of Chemical Information and Modeling, 52(7), 1757–1768. https://doi.org/10.1021/ci3001277
Ito, K., Ito, S., Shimamura, T., Weyand, S., Kawarasaki, Y., Misaka, T., Abe, K., Kobayashi, T., Cameron, A. D., & Iwata, S. (2011). Crystal structure of glucansucrase from the dental caries pathogen Streptococcus mutans. Journal of Molecular Biology, 408, 177–186.
Kartasasmita, R. E., Herowati, R., Harmastuti, N., & Gusdinar, T. (2009). Quercetin derivatives docking based on study of flavonoids interaction to cyclooxygenase-2. Indonesian Journal of Chemistry, 9(2), 297–302.
Kesuma, D., Siswadon, Purwanto, B. T., & Hardjon, S. (2018). Uji in-silico aktivitas sitotoksik dan toksisitas senyawa turunan N-(benzoil)-N’-feniltiourea sebagai calon antikanker. Journal of Pharmaceutical Science and Clinical Research, 3(1), 1–11.
Kriswandini, I. L., Diyatri, I., & Putri, I. A. (2019). Density of Streptococcus mutans biofilm protein induced by glucose, lactose, soy protein and iron. Dental Journal, 52(2), 86–89.
Laskowski, R. A., & Swindells, M. B. (2011). LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. Journal of Chemical Information and Modeling, 51(10), 2778–2786. https://doi.org/10.1021/ci200227u
Malau, N. D., & Azzahra, S. F. (2020). Molecular docking studies of potential quercetin 3,4’-dimethyl ether 7-alpha-L-arabinofuranosyl-(1-6)-glucoside as inhibitor antimalarial. Journal of Physics: Conference Series, 1428, 012057. https://doi.org/10.1088/1742-6596/1428/1/012057
Meng, X. Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: A powerful approach for structure-based drug discovery. Current Computer-Aided Drug Design, 7(2), 146–157. https://doi.org/10.2174/157340911795677602
Nelwan, J. J. (2016). Faktor-faktor yang mempengaruhi tingginya kasus karies gigi pada anak panti asuhan Yataama Al-Firdaus di wilayah kerja Puskesmas Ngesrep tahun 2011. Jurnal Kesehatan Gigi, 3(2), 106–113.
Nigel, B., & Pitts, D. T. Z. P. (2017). Dental caries. Nature Reviews Disease Primers, 3, 17031. https://doi.org/10.1038/nrdp.2017.31
Nur, S., Setiawan, H., Hanafi, M., & Elya, B. (2023). Phytochemical composition, antioxidant, in vitro and in-silico studies of active compounds of Curculigo latifolia extracts as promising elastase inhibitor. Saudi Journal of Biological Sciences, 30(8), 103716. https://doi.org/10.1016/j.sjbs.2023.103716
Nosrati, M., Behbahani, M., Mohabatkar, H., & Shakeran, Z. (2018). Antibacterial and antibiofilm activities of Prangos acaulis Bornm. extract against Streptococcus mutans: An in-silico and in vitro study. Journal of Herbmed Pharmacology, 7(3), 176–184.
Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, e47. https://doi.org/10.1017/jns.2016.41
Patil, R., Das, S., Stanley, A., Yadav, L., Sudhakar, A., & Varma, A. K. (2010). Optimizing hydrophobic interactions and hydrogen bonding at the target–ligand interface leads the pathways of drug-designing. PLOS ONE, 5(8), e12029. https://doi.org/10.1371/journal.pone.0012029
Pratama, M. R. F. (2016). Studi docking molekular senyawa turunan kuinolin terhadap reseptor estrogen-α. Jurnal Surya Medika, 2(1), 1–7.
Ravindranath, P. A., Forli, S., Goodsell, D. S., Olson, A. J., & Sanner, M. F. (2015). AutoDockFR: Advances in protein–ligand docking with explicitly specified binding site flexibility. PLOS Computational Biology, 11(12), e1004586. https://doi.org/10.1371/journal.pcbi.1004586
Ren, Z., Chen, L., Li, J., & Li, Y. (2016). Inhibition of Streptococcus mutans polysaccharide synthesis by molecules targeting glycosyltransferase activity. Journal of Oral Microbiology, 8, 31095. https://doi.org/10.3402/jom.v8.31095
Reokistiningsih, H., Hapsari, D. N., & Almira, H. (2013). Efek ekstrak daun mahkota dewa (Phaleria macrocarpa) sebagai penghambat pembentukan biofilm pada Streptococcus mutans secara in vitro. Prodenta Journal of Dentistry, 1(1), 1–11.
Rollando, R. (2017). Isolasi, identifikasi, karakterisasi, dan uji antibiofilm derivat asam galat dari batang Sterculia quadrifida R. Br. Jurnal Kefarmasian Indonesia, 7(2), 105–111. https://doi.org/10.22435/jki.v7i2.6433105-111
Ruslin, Y., Yana, N. R. A., & Leorita, M. (2020). Desain turunan senyawa leonurine sebagai kandidat obat antiinflamasi. Jurnal Farmasi Galenika, 6(1), 181–191. https://doi.org/10.22487/j24428744.2020.v6.i1.15025
Saxena, M., Saxena, J., Nema, R., Singh, D., & Gupta, A. (2013). Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry, 1(6), 168–182.
Shimamura, A., Nakano, Y. J., Mukasa, H., & Kuramitsu, H. K. (1994). Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. Journal of Bacteriology, 176(16), 4845–4850.
Susanti, N. M. P., Saputa, D. P. D., Hendrayati, P. L., Parahyangan, L. P. D. N., & Swandari, L. A. D. G. (2018). Molecular docking sianidin dan peonidin sebagai antiinflamasi pada aterosklerosis secara in-silico. Jurnal Farmasi Udayana, 7(1), 28–33.
Takahashi, N., & Nyvad, B. (2011). The role of bacteria in the caries process. Journal of Dental Research, 90(3), 294–303. https://doi.org/10.1177/0022034510379602
Towaha, J. (2014). Kandungan senyawa polifenol pada biji kakao dan kontribusinya terhadap kesehatan. Sirinov, 2(1), 1–16.
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461.
Zheng, X., & Polli, J. E. (2010). Identification of inhibitor concentrations to efficiently screen and measure inhibition Ki values against solute carrier transporters. European Journal of Pharmaceutical Sciences, 41(1), 43–52.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Copyright @2017. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-nc-sa/4.0/) which permits unrestricted non-commercial used, distribution and reproduction in any medium